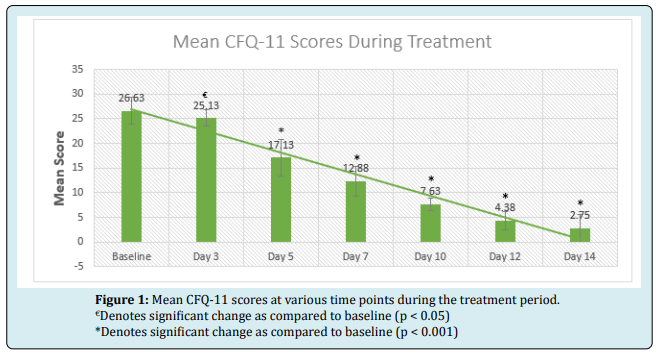
Studies evaluated immune response in both the innate and adaptive immune systems with ImmunoSEB™. In vivo and in vitro studies, as well as clinical trials, measured ImmunoSEB™ as an immunomodulator. The studies also looked at ImmunoSEB™ and immune function and response. They evaluated antibiotic penetration, immunoglobulin formation, and white blood cell (T cell) activity.*

Science & Research
The Research Behind ImmunoSEB™
New COVID-19 Clinical Trial
A new study published in Advances in Clinical Toxicology in February 2021 explored the safety and efficacy of the health supplements ImmunoSEB™ and ProbioSEB CSC3™ (probiotic complex) as supplemental therapy in confirmed mild to moderate COVID-19 patients, finding 93% of patients in the test arm showed clinical improvement on day 10 vs. 60% of patients in the control arm.*
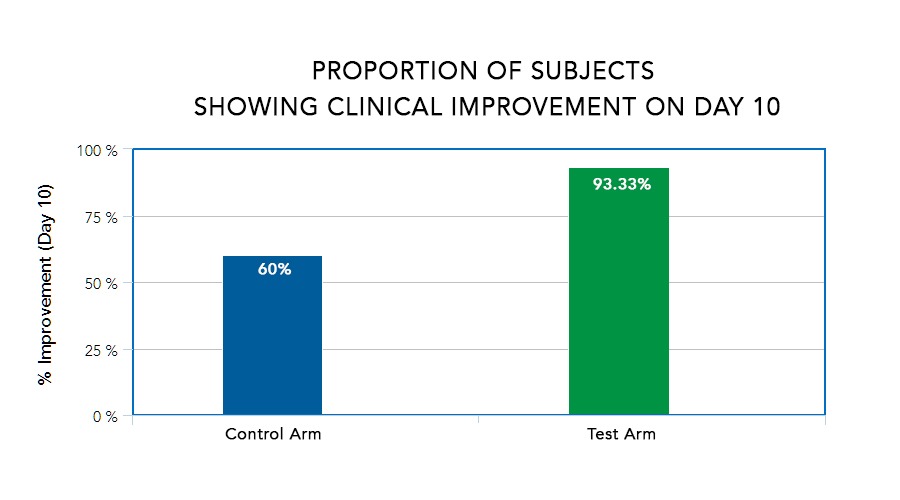
Patients in the test arm had
- a shorter duration of hospitalization
- quicker recovery
- faster reduction in CRP levels
as compared to the control arm. The efficacy and safety of the experimental regimen was compared with the control arm at various time points from days 1 to 21.*
Respiratory Health Clinical Trial
In a randomized, placebo-controlled, single-blind, open-label clinical trial on respiratory health (published in a peer-reviewed journal), 120 sputum-positive participants were studied.
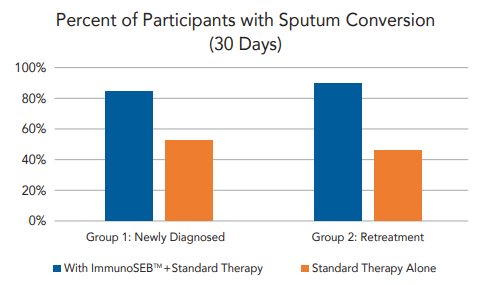
They were divided into two groups:
- newly diagnosed cases (Group 1)
- retreatment cases (Group 2).
Each group was divided, with half the participants receiving standard treatment alone, and the other half receiving ImmunoSEB™ along with the standard treatment.*
Results found sputum conversion occurred faster in participants who received the combo of ImmunoSEB™ and standard treatment, in both Group 1 and Group 2. It was especially significant in Group 2, or retreatment patients who may have built up drug-resistance.*
Resistance & Retreatment
In Vivo Studies
ImmunoSEB™ was studied in vivo both healthy and immunosuppressed animals. Antibiotic efficacy, and immunity markers such as T-cells and Immunoglobulin G (IgG), were evaluated.
In Vitro Studies

ImmunoSEB™ was studied in vitro on several clinically significant pathogenic fungi and bacterias, both solo and in combination with antibiotics.*
ImmunoSEB™ demonstrated antifungal activity by inhibiting fungi such as Candida albicans and filamentous fungi Aspergillus niger. ImmunoSEB™ also showed synergy with antifungal medications commonly used to treat five clinically significant fungi. It was demonstrated by a drastic drop in the minimum inhibition concentration (MIC) values of standard antifungals.*
ImmunoSEB™ was screened for in vitro antibacterial activity using ATCC strains and clinical isolates of Salmonella typhi, Pseudomonas aeruginosa, E. coli, Staphylococcus aureus, Streptococcus pyogenes and S. pneumoniae. It also showed bacteriostatic activity against E.coli and Salmonella.*
ImmunoSEB™ was found to have broad-range antibacterial activity against several commonly encountered pathogens, as well as with some of their clinical isolates. It also showed synergy with specific antibiotics.*
References
- Joshi A. et. al., Screening for antifungal activity of polyenzyme preparation ImmunoSEB™-S using in vitro methods, Trends in Biosciences 6 (3), 234- 236, 2013.
- Joshi A. et. al., In vitro evaluation of antibacterial activity of a novel polyenzyme preparation ImmunoSEB™, Trends in Biosciences 6 (2), 142 – 145, 2013.
- Koyana A. et. al., Augmentation by serrapeptase of tissue permeation by Cefotiam, Japanese Journal of Antibiot. 39 (3), 761 – 771, 1986.
- Joshi A. et. al., Determination of antimycobacterial activity of polyenzyme preparation ImmunoSEB™-S using in vitro methods, Trends in Biosciences 5 (1), 61 – 63, 2012.
- Mohanty K. C. et. al., Efficacy of Lysozyme – Lactoferrin as a bioenhancer (immunomodulator) in the treatment of Tuberculosis, RGUHS Journal of Medical Sciences, 2 (2), 91 – 95, 2012.
- See page 4 of ImmunoSEB™ White Paper, Performance in In Vivo Study (unpublished).
- SIQUEIROS-CENDÓN T et. al., Immunomodulatory effects of lactoferrin, Acta Pharmacologica Sinica, 35: 557–566, 2014.
- Giansanti F et. al., Lactoferrin from Milk: Nutraceutical and Pharmacological Properties, Pharmaceuticals, 9, 61, 2016.
- Ragland S A et. al., From bacterial killing to immunemodulation: Recent insights into the functions of lysozyme, Pathogens 13(9), 2017.
- Hunghey V L, et. al., Antimicrobial Activity of Lysozyme against Bacteria Involved in Food Spoilage and Food-Borne Disease, Applied and Environmental Microbiology, Vol. 53, 9, 2165 – 2170, 1987.
- Selan L et. al., Serratiopeptidase reduces the invasion of osteoblasts by Staphylococcus aureus, International Journal of Immunopathology and Pharmacology, Vol. 30(4) 423–428, 2017.
- Tiwari M, The role of serratiopeptidase in the resolution of inflammation, Asian Journal of Pharmaceutical Sciences, Volume 12, 3, 209-215, 2017.